It is time to convince the Food and Drug Administration (FDA) to regulate fluoridated waters and chemicals as drugs. On June 1, 2015, ten organizations, including Food and Water Watch and Fluoride Action Network, joined together to submit a Petition to FDA requesting adoption of a regulation that would make these waters and chemicals both drugs regulated by FDA to ensure that they are safe and effective. The Petition was assigned docket number FDA-2015-P-1977 and FDA is accepting your comments in support of the Petition. To read the Petition left click on this hyperlink: http://www.
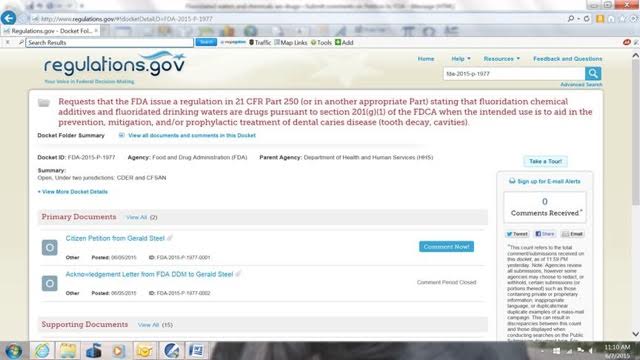
When you get this page, left click on any of the “Open Docket Folder” links that will be on the right side of your page. You will get a page similar to this:

Note that Petition content is summarized by FDA in red at the top of this page and states that the Petition:
- Requests that the FDA issue a regulation in 21 CFR Part 250 (or in another appropriate Part) stating that fluoridation chemical additives and fluoridated drinking waters are drugs pursuant to section 201(g)(1) of the FDCA when the intended use is to aid in the prevention, mitigation, and/or prophylactic treatment of dental caries disease (tooth decay, cavities).
When you get this page, you can read the 10-page Citizen Petition [signed by] Gerald Steel on behalf of ten organizations by left clicking once on “Citizen Petition from Gerald Steel.” On the next page that opens, left click on the pdf symbol. On Windows 7, you will likely get a box on the bottom of the page asking if you want to “Open” or “Save” the file. Left click “Open.” The pdf of the Petition will then be on your screen for you to read. On Windows 7, you can left click the file button in the upper left corner and choose to print or save this document.
When you have finished with the Petition or if you just want to comment based on the FDA Summary, start with the page similar to the page pasted in above and you left click once on the box that says “Comment Now!” and a page similar to the page pasted below will open:
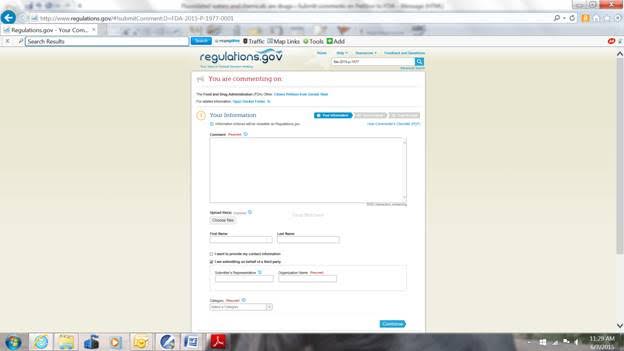
If you want to submit a letter or other documents as attachments, left click on the “Choose files” box under the words “Upload file(s)” just under the “Comment” box. You can then browse on your computer for your files. Your attachments must be in Word or in pdf files. You can attach multiple files of mixed Word and pdf types but the maximum size of all attached files in one Comment Submittal is 10 MB. You can submit multiple Comment Submittals but you have to start over from the beginning for each one. You can type in your First and Last names or, if you don’t, your comment will filed under “Anonymous.” You can click either, neither or both boxes below the first and last name boxes. If you left click in the box for “I want to provide my contact information” a box will open up for you to give your state or province, country, zip code, and email address (whichever apply). If you leave the box for “I am submitting on behalf of a third party” checked, then fill in the requested information, or otherwise uncheck the box. Finally, on this page you must select a category for the person or organization making the comment. Popular are “individual consumer” or “other organizations.” Then left click the “Continue” box on the lower right corner and a new page will open for you to preview your Comment Submittal.
You can type your comment into the “Comment” box or you can paste a text-only comment into the “Comment” box (with a maximum of 5000 total characters). You have to put something into the “Comment” box even if it is only a description of your attachments. When other people access this docket, they will see what you put in the “Comment” box, they will be able to read any attachments, and you will only be identified by your country, state or province, and your “category” which I will explain below.
To make edits, click the “Back” button (upper left corner) or the “Edit” box (lower right corner) to return to the previous page. Make the edits, and again click the “Continue” box. When you are satisfied with your Comment Submittal, check the box on the lower center part of the page next to the words, “I read and understand the statement above.” Then in the lower right corner, click the “Submit Comments” box. A page should open with a Receipt saying, “Your comment was submitted successfully.”
I recommend that you print this Receipt because it has your comment tracking number or better, if you gave your email address, you can click the box “Email Receipt” and you will get even better information in an email.
